Losing weight is a longstanding goal for many Americans, and many are quick to jump at any new options that promise to make that goal more achievable. Public interest in weight-loss drugs is at an all-time high as social media influencers and mainstream media outlets put a spotlight on newer medications that can help with significant weight loss. Still, many questions about these medications remain unanswered.
The most highly discussed drugs of the moment – known in the industry as “incretin mimetics” – were originally approved to treat diabetes, but have also shown benefits with weight loss. The drugs getting the most attention are semaglutide (Ozempic, Wegovy) and to a lesser extent tirzepatide (Mounjaro), but they aren’t alone in their drug class. As seen in the Google Search Trend chart from February 2023 below, public interest in these drugs has spiked over the last six months.
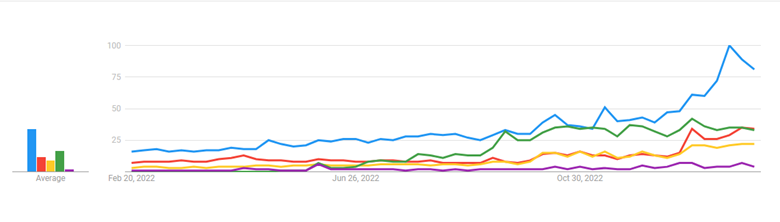
Blue: Ozempic; Red: Wegovy; Yellow: semaglutide; Green: Mounjaro; Purple: weight loss drug
Data Source: Google Trends (https://www.google.com/trends). Accessed 2/14/22
Do these drugs help with weight loss?
As a whole, yes. While not everyone responds to these medications for weight loss, in clinical trials of Wegovy for weight loss, patients lost between 10% and 17.5% of their body weight, compared with either a weight gain or up to 5% weight loss without the treatment.1 The other drugs in this class approved for diabetes also showed positive effects on weight, with Mounjaro being the most effective. Mounjaro isn’t approved for weight loss as of this writing, but clinical trial data shows average weight loss over 15%, with most patients losing over 20% of their body weight at the highest dose.2 The manufacturer for Mounjaro received a “fast track” designation from the FDA for an approval to use Mounjaro to treat weight-related conditions.3 A decision from the FDA (likely an approval based on the data available) is expected sometime in 2023 or 2024.
If some of these drugs have the same active ingredient – semaglutide (Ozempic, Wegovy) and liraglutide (Victoza, Saxenda), for example – and the same mechanism of action, why would they be approved for different uses? Ozempic and Victoza are FDA-approved to treat type 2 diabetes, while Wegovy and Saxenda are FDA-approved for chronic weight management. They’re the same drug but dosed and studied slightly differently, leading to different FDA approvals – and different insurance coverage. Many plans don’t cover medications approved for obesity, which is causing increased off-label use for the drugs approved for diabetes.
Injectable Incretin Mimetics |
||
| Active Ingredient | Brand Name Product | FDA Approval |
| Semaglutidea | Ozempic | Diabetes |
| Semaglutidea | Wegovy | Weight management |
| Liraglutidea | Victoza | Diabetes |
| Liraglutidea | Saxenda | Weight management |
| Dulaglutidea | Trulicity | Diabetes |
| Exenatidea | Bydureon | Diabetes |
| Tirzepatideb | Mounjaro | Diabetes |
a – GLP-1; b – GIP/GLP-1
What’s the impact of the media hype?
The increased media attention on these drugs has pros and cons. It has highlighted that being overweight or obese is a chronic health condition that’s treatable with effective drugs, which has been a challenging public and medical perception issue for many years.6 Also, many people will likely get treatment they didn’t otherwise realize was available to help them lose weight successfully.
However, increased off-label use of the drugs for diabetes (Ozempic, Mounjaro, Trulicity, etc.) to help lose weight has led to shortages of medication for those who need it to manage diabetes.7 This causes treatment delays and increases in blood sugar, and it impairs adherence to the treatment. Supply issues may also require patients to go to other pharmacies where they don’t have a relationship with their pharmacist, and drug-drug interactions could be missed due to lack of a full medication profile to review. And since these drugs also require gradual dose titration when starting treatment, patients may have to re-titrate their dose if they go without the medication for weeks at a time.
The bottom line
There are always two sides of the coin with using medications to treat disease, and weight management is no different. On the whole, these popular new drugs are showing themselves to be highly effective and easy to use, with an overall good safety and tolerability profile. But they aren’t without their drawbacks – not everyone responds to treatment, and some patients have experienced side effects that include GI, pancreatitis, and gallbladder issues. These drugs also come with the same caveat as any other drug used for weight loss: Weight loss is hard, and any medication used without a behavior change program will lead to weight regain in many patients.
And the off-label use of diabetes drugs for weight-loss purposes raises even more concerns. Without FDA approval for weight loss, it’s unclear how safe and effective the diabetes drugs will be for long-term weight management. And some may question the ethics of using those drugs for weight loss as the diabetes patients who were the intended recipients now struggle to get the medications they need. Clinical oversight is critical to ensure medications are appropriately prescribed for the health and safety of the member taking them.
References
- McDermid E. A quick guide to the STEP trials. Medicine Matters. https://diabetes.medicinematters.com/semaglutide/obesity/quick-guide-step-trials/18854832. Published February 2, 2021. Updated November 2022. Accessed February 10, 2023.
- McDermid E. A quick guide to the SURPASS and SURMOUNT trials. Medicine Matters. https://diabetes.medicinematters.com/tirzepatide/type-2-diabetes/a-quick-guide-to-the-surpass-and-surmount-trials/18478154. Published October 14, 2020. Updated August 2022. Accessed February 10, 2023.
- Eli Lilly and Company. Lilly receives U.S. FDA Fast Track designation for tirzepatide for the treatment of adults with obesity, or overweight with weight-related comorbidities. Lilly Investors. https://investor.lilly.com/news-releases/news-release-details/lilly-receives-us-fda-fast-track-designation-tirzepatide. Published October 6, 2022. Accessed February 22, 2022.
- Kaneko S. Tirzepatide: A Novel, Once-weekly Dual GIP and GLP-1 Receptor Agonist for the Treatment of Type 2 Diabetes. touchREV Endocrinol. 2022;18(1):10-19. doi:10.17925/EE.2022.18.1.10
- Wegovy Savings Offer. NovoCare.com. https://www.novocare.com/wegovy/savings-card.html. December 2022. Accessed February 10, 2023.
- Bendix A. Ozempic and Wegovy add new layers to the understanding of obesity as a chronic health condition. NBC News. https://www.nbcnews.com/health/health-news/ozempic-wegovy-obesity-chronic-health-condition-rcna68831. February 8, 2023. Accessed February 10, 2023.
- ASHP Drug Shortages List – Current Drug Shortage Bulletins. American Society of Health-System Pharmacists. https://www.ashp.org/drug-shortages/current-shortages/drug-shortages-list?page=CurrentShortages. Accessed February 10, 2023.





